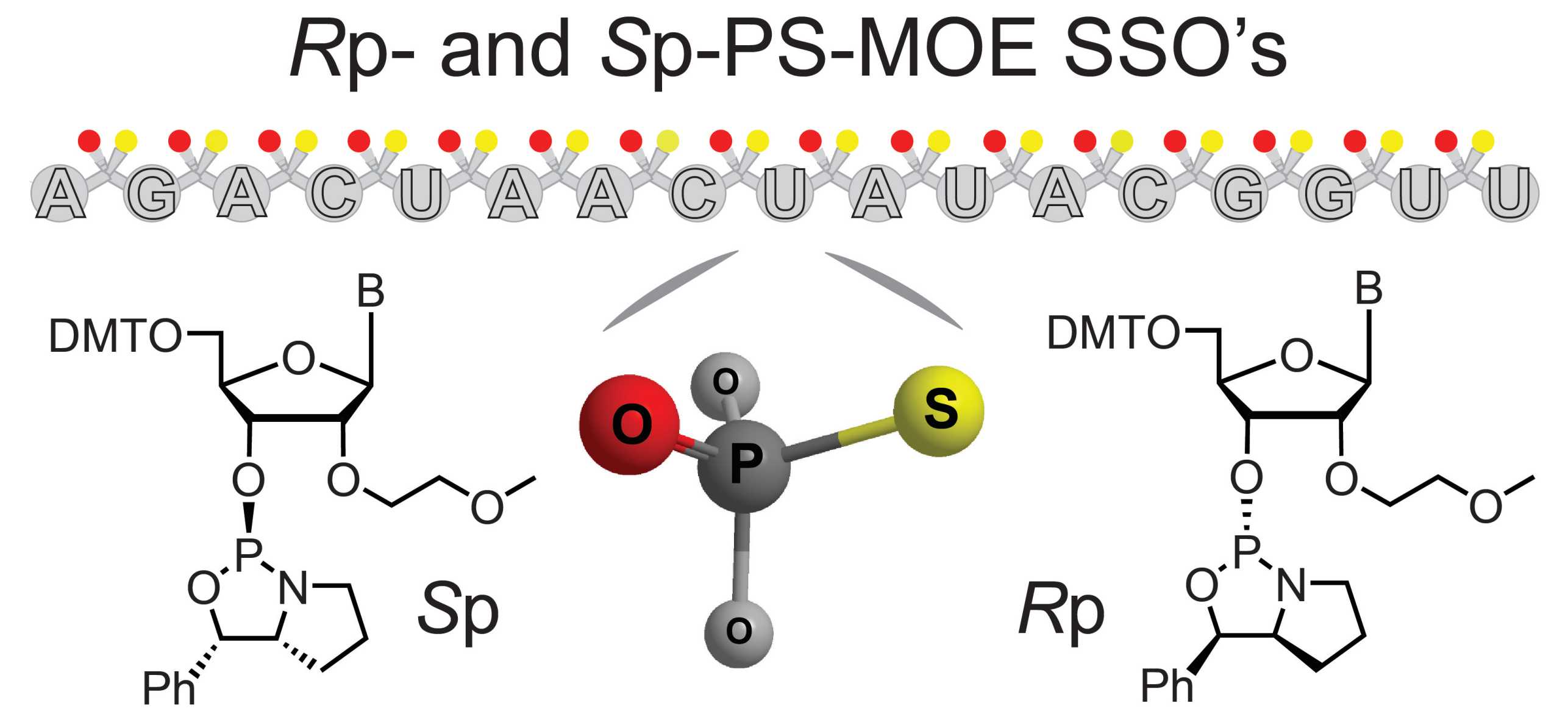
Phosphorothioate stereochemistry in oligonucleotide drugs
A spate of recent approvals for oligonucleotide drugs in the last few years has rewarded perseverance of the field. These drugs are isomeric mixtures due to their chiral phosphorothioate (PS) linkages.
In Stereochemical bias introduced during RNA synthesis modulates the activity of PS-siRNAs (external page Nat Commun 2015), we described an unexpected observation that low levels of stereoselectivity in coupling during solid phase synthesis produced additive effects in the biophysical properties of siRNAs, and affected their properties in cells.
We later extended the investigation to single-stranded PS-oligonucleotides (external page Chem Commun 2017), and published the first account of cellular activity from stereopure reagents.
Few groups are able to make these molecules and our patent application appears to be robust. This is especially important given that stereopure PS-drugs likely represent the future of oligonucleotide therapeutics.